
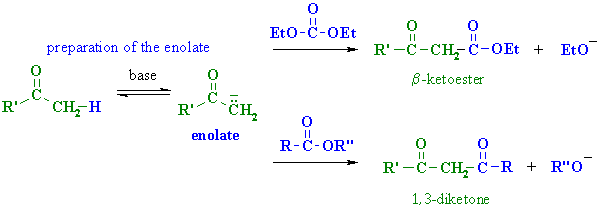
The reaction rate will be determined by the visual indicator in the reaction. All reactions have the same general reaction scheme:Īll rates of the reactions are relative to each other. In part one, we will study the S N1 reaction using 11 test tube reactions. In two parts, we will examine the factors that affect the relative rates of the S N1 and S N2 reactions. The product is still chiral, but now is an even mixture of both enantiomers which is called a racemic mixture. Since the carbocation is achiral (not chiral), any stereochemistry at the beginning of the reaction is lost. In the second step, the nucleophile attacks the carbocation. In the first step, the leaving group leaves and forms an intermediate called a carbocation.
This is an example of a stereospecific reaction where the stereochemistry at the onset of the reaction dictates the outcome of the stereochemistry after the reaction.Ī two-step nucleophilic substitution is an example of the S N1 mechanism. This back-side attack inverts the stereochemistry on the reacting carbon. Therefore, some nickname the S N2 reaction the "back-side attack". One thing to keep in mind is that the molecular orbitals involved in the reaction indicate that the nucleophilic attack must come at 180 ° from the leaving group. This reaction is a concerted reaction where the bonds breaking and forming are occurring at the same time. A one-step nucleophilic substitution is an example of the S N2 mechanism. In a nucleophilic substitution reaction, a nucleophile replaces a leaving group on a carbon atom.


 0 kommentar(er)
0 kommentar(er)
